Fuel oil is an important energy source derived from petroleum and is used as fuel in various fields of industry, as well as transportation. the depletion of petroleum reserves, also cannot be renewed, Currently, many people are competing to find alternative energy from petroleum that can be renewed so that its availability can be guaranteed.
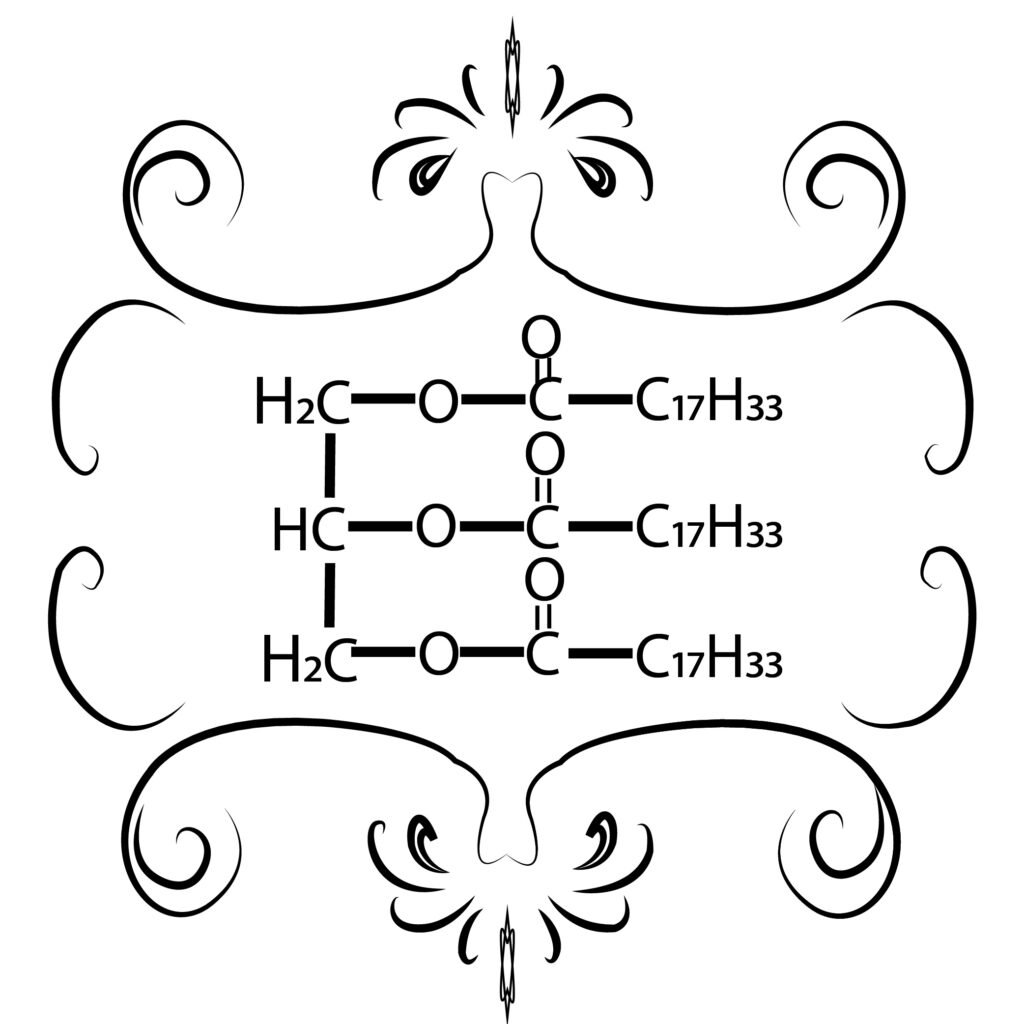
Biodiesel is a chemical process in the form of methyl and ester compounds from the esterification and transesterification process of vegetable oils or animal fats or one of the alternative fuels produced from natural and renewable materials, such as palm oil, castor oil, soybean oil, while examples of animal fats include beef fat, lard, and other animal fats. Biodiesel is usually made from the esterification/transesterification process of vegetable oils or animal fats using alcohol, Zeolites here have a role as a catalyst or help speed up chemical reactions. The use of biodiesel has several advantages compared to using diesel oil as follows:
1.) The use of biodiesel is more environmentally friendly when compared to fuel from petroleum because it produces better emissions.
2.) has a higher cetane number (>57) so that the combustion process is better
3.) has lubricating properties against engine pistons
4.) biodiesel can be renewed or produced continuously because the raw material for biodiesel uses renewable vegetable oils or animal fats. 5.) increase to supply of own fuel because it can be produced locally.

Materials that can be used for the manufacture of biodiesel can use compounds contained in pork fat or others in the form of triglycerides, triglycerides when reacted with alcohol will produce esters and glycerol. This process is often called a transesterification reaction. While the esterification reaction is a nucleophilic ion substitution reaction with an acid catalyst. Homogeneous catalysts are catalysts that are soluble in the reactants or reaction products, while heterogeneous catalysts are catalysts that are different from the reactants or products. Heterogeneous catalysts have several advantages compared to homogeneous catalysts, including having high catalytic activity, being more stable during transesterification or esterification, and being is easily separated.

Zeolite is a heterogeneous catalyst and contains cations K+ (Potassium), Na+ (Sodium), Ca2+ (calcium), and Mg2+ (Magnesium) while synthetic zeolite usually only contains K+ (calcium), and Na+ (Sodium). Zeolite has a hollow structure or pores, usually, this cavity is widely used as an adsorbent, filter, natural cation exchanger, and used as a catalyst. The advantages of using clinoptilolite natural zeolite as a catalyst in the manufacture of biodiesel :
1.) clinoptilolite natural zeolite has a solid form and cavity, so zeolite can be very suitable to be used as a catalyst.
2.) able to increase ester purity.
3.) Easier to separate than a liquid catalyst.
the use of zeolites can also streamline the process of acid esterification and base transesterification where the process is carried out using zeolites can be done simultaneously or not separately to save time, energy, and others.
From the explanation above, it can be concluded that the use of zeolite as a catalyst is very helpful in making very practical biodiesel.





